 |
||
|
|
||
|
|
 |
 ROOM TEMPERATURE WET CHEMICAL
GROWTH (RTWCG) PROCESS
ROOM TEMPERATURE WET CHEMICAL
GROWTH (RTWCG) PROCESS
The RTWCG process comprises soaking the Si substrates into the growth solution. The process utilizes a mixture of inexpensive liquid Si, C, F, and N precursors, and homogeneous catalysts are used to increase the growth rate. In order to adjust the pH of the growth solution, non-invasive alkaline solutions are added to the growth system. The liquid precursors used thus far are of technical grade purity, e.g. averaging about 99%. Surface OH groups are known in the art as one of the most important sites for chemical reactions at oxide surfaces. The OH groups are formed by the chemisorption of water molecules on the oxide surface. The hydration mechanism involves the dissociation of an adsorbed water molecule, where an H+ ion bonds to an oxygen ion on the surface and an OH- ion bonds to a silicon ion on the surface. The SiOH groups can undergo acid or base reactions. They accept a hydrogen ion to become a SiOH2+ site having a positive charge, or they release a hydrogen ion to become SiO- site having a negative charge. The chemical reactions are written as:
SiOH + H+(Aq)
SiOH The concentration of the SiOH2+ and SiO- species depends on the pH of the aqueous phase. The SiOH2+ species increases at pH<7, while the SiO- species increases at pH>7. After the RCA clean and prior to the final water rinse, the silicon surface is passivated by Si-H and Si-F bonds. We define the "induction time" as the time interval dt = tox - tin, where tox is the time referenced to the initial time (tin) after which the oxide deposition is initiated. For HF treated surfaces we found an induction time from 10 seconds to 2 minutes. Prior to the initiation of the RTWCG of SiOX-based layer, for hydrogen- or fluorine-terminated Si surfaces the Si-H and Si-F bonds has to be converted into the Si-OH bonding group. The hydration chemical mechanism of hydrogen terminated surfaces should follow the reaction:
Si-H + OH-
For Si-F terminated bonds,
rinsing the samples in water allows the Si-F
Si-F + H2O From the last two reactions, it is apparent that in the presence of HF, the surface may be subject to HF attack through HF insertion into the Si-O bond, according to the reaction:
Si-O- + HF with the subsequent removal of the surface Si atom from the surface of the underlying Si-O oxide. We found that after the HF dip, rinsing the substrates in deionized water for 5 to 10 minutes or into 0.1% H2O2 for 1 to 2 minutes, depending on the crystallographic orientation, doping type and majority carrier concentration, the induction time for all silicon substrates was below 20 seconds. Once the growth of a SiO-based oxide layer has began, Si-H and Si-F bonds are replaced by the Si-OH bonds. These Si-OH groups in turn facilitate the incorporation from the growth solution of oxygen by forming Si-O-Si bonds. Associated Si-OH groups also act as preferential adsorption sites for water molecules further speeding up oxygen and silicon incorporation process. |
© 2009 SPECMAT, Inc. All Rights Reserved |
||
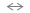
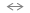 SiO
SiO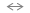 Si-O
Si-O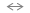 Si-OH to take place according
to the reaction:
Si-OH to take place according
to the reaction: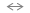 Si-OH +
HF
Si-OH +
HF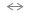 Si-F + OH
Si-F + OH